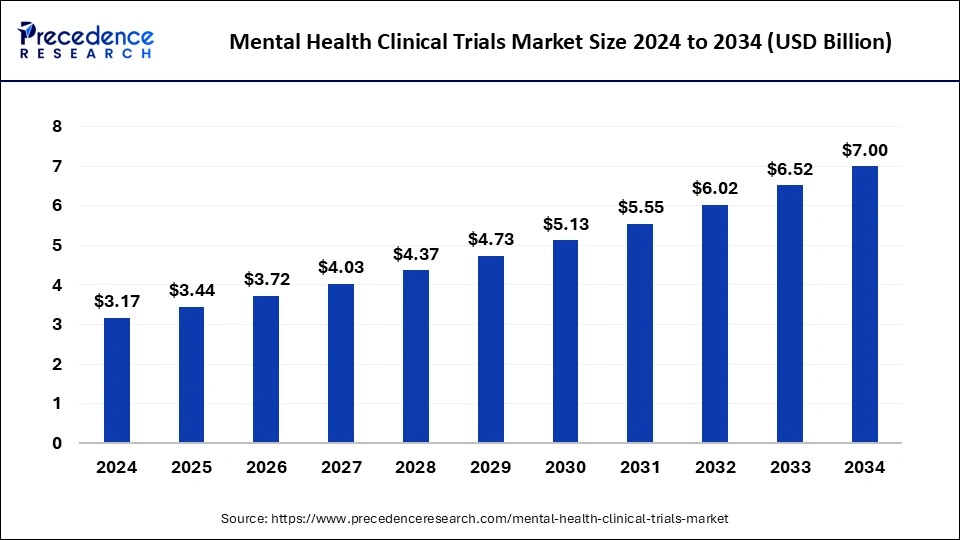
The global mental health clinical trials market size is expected to increase USD 6.52 billion by 2033 from USD 2.93 billion in 2023 with a CAGR of 8.32% between 2024 and 2033.
Mental Health Clinical Trials Market Key Points
- North America dominated the mental health clinical trials market with the largest revenue share of 51% in 2023.
- Asia Pacific is expected to grow at the highest CAGR of 8.82% during the forecast period.
- By phase, the phase III segment has contributed more than 33% of revenue share in 2023.
- By phase, the phase I segment is projected to grow at the solid CAGR of 8.71% during the forecast period.
- By study design, the interventional segment has captured more than 66% of revenue share in 2023.
- By study design, the observational segment is expected to grow at the highest CAGR in the market during the forecast period.
- By sponsor, the pharmaceutical & biopharmaceutical companies segment has recorded the biggest revenue share of 40% in 2023.
- By sponsor, the government agencies segment is growing at a highest CAGR of 8.53% during the forecast period.
- By disorder, the anxiety disorders segment dominated the market in 2023.
- By disorder, the depression segment is expected to grow at the highest CAGR in the market during the forecast period
The mental health clinical trials market is an essential segment of the broader healthcare and pharmaceutical industry. Mental health disorders, including depression, anxiety, bipolar disorder, schizophrenia, and other psychiatric conditions, are prevalent worldwide, affecting millions of people and significantly impacting their quality of life. The clinical trials for mental health are designed to explore new treatments, understand the efficacy and safety of existing medications, and investigate novel therapeutic approaches. These trials are crucial for advancing mental health care and providing evidence-based treatments.
The market is driven by the increasing prevalence of mental health disorders, growing awareness about mental health issues, and the rising demand for effective treatment options. Additionally, advancements in neuroscience and pharmacology have paved the way for the development of innovative therapies and medications, further fueling the growth of the market. Regulatory bodies like the FDA and EMA have also recognized the urgent need for effective mental health treatments, resulting in more streamlined approval processes for new drugs.
Get a Sample: https://www.precedenceresearch.com/sample/4587
Growth Factors
Several factors contribute to the growth of the mental health clinical trials market. Firstly, the rising incidence of mental health disorders globally has created a pressing need for new and effective treatments. According to the World Health Organization (WHO), depression alone affects more than 264 million people worldwide, and this number is expected to increase due to various socio-economic and environmental factors.
Secondly, there has been a significant increase in funding and investments in mental health research. Governments, non-profit organizations, and private entities are allocating substantial resources to understand and address mental health issues. This financial support is crucial for conducting large-scale clinical trials and advancing mental health research.
Thirdly, the stigma surrounding mental health is gradually decreasing, leading to more individuals seeking help and participating in clinical trials. Public awareness campaigns and educational initiatives have played a vital role in changing perceptions and encouraging people to discuss their mental health openly.
Moreover, technological advancements have revolutionized the way clinical trials are conducted. Digital health tools, telemedicine, and remote monitoring devices have made it easier to recruit and retain participants, collect data, and ensure compliance. These innovations have not only improved the efficiency of clinical trials but also expanded their reach to diverse populations.
Region Insights
North America, particularly the United States, dominates the mental health clinical trials market. The region’s leadership is attributed to its robust healthcare infrastructure, high prevalence of mental health disorders, and substantial funding for mental health research. The National Institute of Mental Health (NIMH) in the U.S. plays a pivotal role in supporting clinical trials and advancing mental health research. Furthermore, the presence of major pharmaceutical companies and academic institutions in the region fosters innovation and collaboration in mental health clinical trials.
Europe is another significant market for mental health clinical trials. Countries like the United Kingdom, Germany, and France are at the forefront of mental health research and clinical trials. The European Union’s regulatory framework for clinical trials, coupled with strong support from national governments and non-profit organizations, has facilitated the growth of the market. Additionally, the increasing focus on mental health in European healthcare policies has led to more investments in research and development.
The Asia-Pacific region is witnessing rapid growth in the mental health clinical trials market. The rising prevalence of mental health disorders, coupled with increasing awareness and access to healthcare, has driven the demand for clinical trials. Countries like China, Japan, and India are emerging as key players in the market, with substantial investments in mental health research. The region also benefits from a large and diverse patient population, which is essential for conducting comprehensive clinical trials.
Mental Health Clinical Trials Market Scope
| Report Coverage | Details |
| Market Size by 2033 | USD 6.52 Billion |
| Market Size in 2023 | USD 2.93 Billion |
| Market Size in 2024 | USD 3.17 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 8.32% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Phase, Study Design, Sponsor, Disorder, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Mental Health Clinical Trials Market Dynamics
Drivers
Increasing Prevalence of Mental Health Disorders
The growing burden of mental health disorders is a primary driver of the clinical trials market. Conditions such as depression, anxiety, bipolar disorder, and schizophrenia are becoming increasingly common, necessitating the development of new and effective treatments. The high prevalence of these disorders has created a significant demand for clinical trials to explore novel therapeutic approaches and medications.
Advancements in Neuroscience and Pharmacology
Advancements in neuroscience and pharmacology have revolutionized the understanding and treatment of mental health disorders. Breakthroughs in brain imaging, genetic research, and neuropharmacology have paved the way for the development of targeted therapies and personalized medicine. These advancements have expanded the scope of clinical trials and enabled researchers to explore innovative treatment options.
Regulatory Support and Fast-track Approvals
Regulatory bodies such as the FDA and EMA have recognized the urgent need for effective mental health treatments. As a result, there are more streamlined approval processes and incentives for developing drugs for mental health disorders. Fast-track designations, priority reviews, and orphan drug status have accelerated the approval of new medications, encouraging pharmaceutical companies to invest in mental health clinical trials.
Opportunities
Development of Personalized Medicine
The growing understanding of the genetic and biological basis of mental health disorders has opened up opportunities for the development of personalized medicine. By tailoring treatments to the individual characteristics of patients, researchers can improve the efficacy and safety of mental health interventions. Clinical trials focused on personalized medicine have the potential to revolutionize mental health care and provide more effective treatments.
Integration of Digital Health Tools
The integration of digital health tools and technologies presents significant opportunities for the mental health clinical trials market. Mobile applications, wearable devices, and telemedicine platforms can enhance participant engagement, data collection, and monitoring. These tools enable remote assessments, real-time data capture, and continuous monitoring, improving the accuracy and efficiency of clinical trials. Additionally, digital health tools can facilitate the recruitment of diverse and geographically dispersed populations, ensuring more representative and inclusive trials.
Expansion into Emerging Markets
Emerging markets, particularly in Asia-Pacific, Latin America, and the Middle East & Africa, offer substantial growth opportunities for the mental health clinical trials market. The rising prevalence of mental health disorders, increasing awareness, and improving healthcare infrastructure in these regions create a favorable environment for conducting clinical trials. Collaborations with local research institutions and healthcare providers can facilitate the expansion of clinical trials into these markets, enabling access to diverse patient populations and accelerating the development of new treatments.
Challenges
Recruitment and Retention of Participants
Recruiting and retaining participants for mental health clinical trials can be challenging. The stigma associated with mental health disorders, concerns about confidentiality, and the burden of participation can deter individuals from enrolling in trials. Additionally, the nature of mental health conditions, which often fluctuate over time, can make it difficult to maintain participant engagement throughout the trial duration. Addressing these challenges requires effective communication, patient-centric approaches, and the use of digital tools to enhance participant recruitment and retention.
Ethical Considerations
Ethical considerations are paramount in mental health clinical trials. Researchers must ensure the protection of participants’ rights, safety, and well-being. Informed consent, privacy, and confidentiality are critical aspects that need to be carefully addressed. Vulnerable populations, such as individuals with severe mental health conditions, require special attention and additional safeguards. Adhering to ethical guidelines and maintaining transparency is essential to build trust and ensure the integrity of mental health clinical trials.
Read Also: Blockchain AI Market Size to Worth USD 3,718.34 Mn by 2033
Mental Health Clinical Trials Market Companies
- ICON Plc
- Eli Lilly Company
- Caidya
- Syneous Health
- Novo Nordisk
- Pharmaceutical Product Development, LLC
- Parexel International Corporation
- Corcept
- Labcorp Drug Development
- IQVIA
Recent Developments
- In April 2024, the international pharmaceutical business Otsuka Pharmaceutical Development & Commercialization, Inc. (Otsuka) announced the opening of My Mental Health Journey, a longitudinal registry project with the goal of advancing mental health and depression research.
- In February 2024, the Bazouki Group declared the beginning of a new collaboration on a clinical study of a therapeutic ketogenic diet for bipolar illness with McLean Hospital in Belmont, Massachusetts. Investigating nutritional ketosis as a unique therapeutic approach and a crucial tool for comprehending the underlying processes of bipolar disease.
Segment Covered in the Report
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Interventional
- Observational
- Others
By Sponsor
- Pharmaceutical & Biopharmaceutical Companies
- Government Agencies
- Others
By Disorder
- Anxiety Disorders
- Depression
- Bipolar Affective Disorder
- Dissociation & Dissociative Disorders
- Schizophrenia
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/