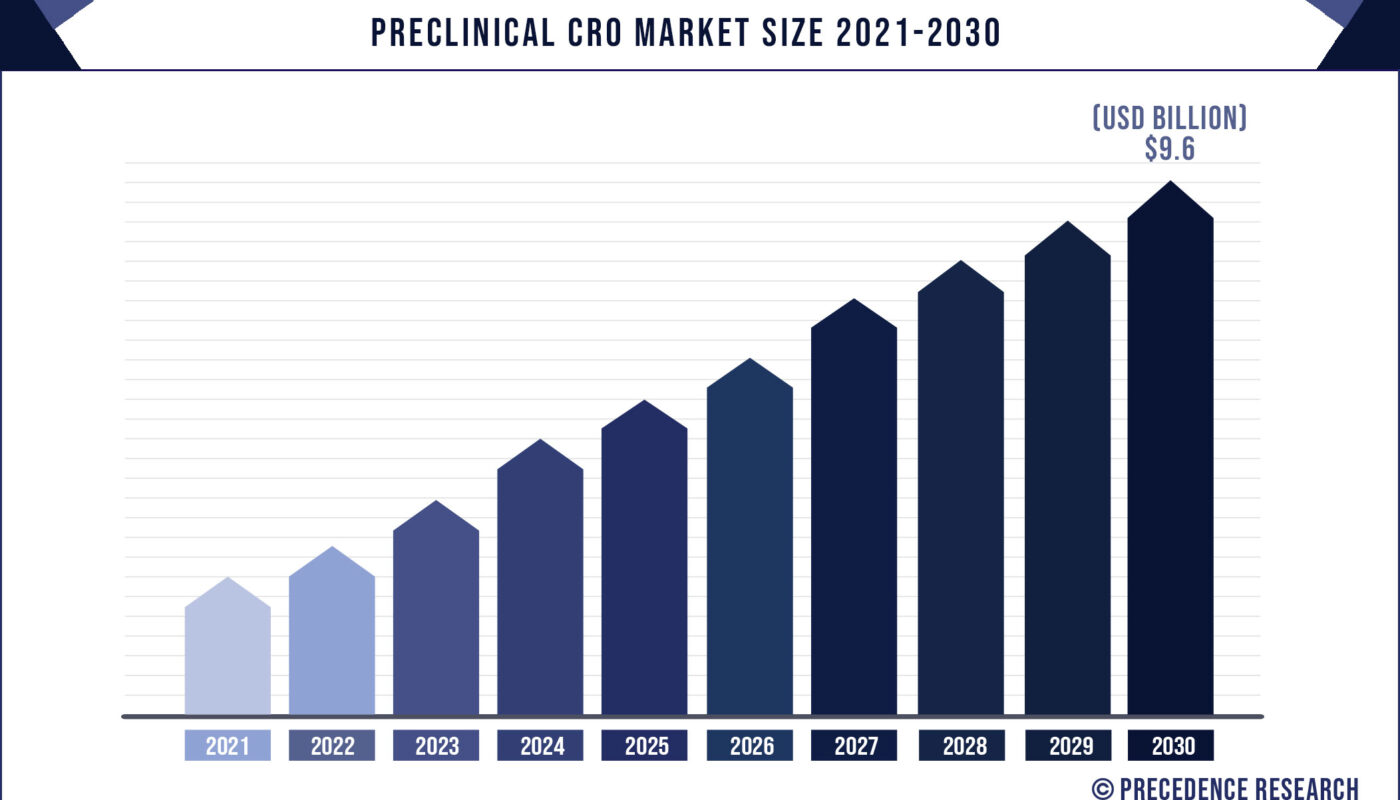
According to the industry experts, The global preclinical CRO market was valued at USD 4.9 billion in 2021 and is projected to reach US$ 9.6 billion by 2030, growing at a CAGR of 7.5% from 2021 to 2030. The report contains 150+ pages with detailed analysis.
The base year for the study has been considered 2021, the historic year 2019 and 2020, the forecast period considered is from 2021 to 2030. The preclinical CRO market is analyzed on the basis of value (US$ Million), volume (Unit), and price (US$/Unit).
The global preclinical CRO market is primarily driven by the rapid growth of the pharmaceutical and the medical devices companies. The rising burden of numerous chronic diseases along with the increasing need for their diagnosis, prevention, and treatment related drugs and devices is significantly driving the market. The various biopharmaceutical companies and academic institutions areincreasingly investing and outsourcing the preclinical studies activities to the CROs owing to the increasing need for the development of new and innovative medicines. The favorable government policies regarding the development of preclinical studies of various diseases and its cure are driving the demand for the preclinical CRO services across the globe.
The recent outbreak of the COVID-19 pandemic compelled the pharmaceutical industry players and research institutions to infuse investments in the CROs to conduct the research activities regarding the COVID-19 virus. Therefore, rising investments in the preclinical CROs by various stakeholders is expected to drive the growth of the global preclinical CRO market.
Download the Sample Pages of this Report for Better Understanding (Including TOC, List of Tables & Figures, and Chart) @ https://www.precedenceresearch.com/sample/1361
Preclinical CRO Market Scope
This market report studies market dynamics, status and outlook especially in North America, Europe and Asia-Pacific, Latin America, the Middle East and Africa. This research report offers scenario and forecast (revenue/volume), and categorizes the market by key players and various segment. This report also studies global market prominence, competitive landscape, market share, growth rates market dynamics such as drivers, restraints and opportunities, and distributors and sales channels.
This research study also integrates Industry Chain analysis and Porter’s Five Forces Analysis. Further, this report offers a competitive scenario that comprises collaborations, market concentration rate and expansions, mergers & acquisitions undertaken by companies.
The rapidly growing number of biopharmaceutical companies across the globe, especially in the developed and developing regions is deriving the demand for the preclinical CRO services. Mostly, the small and the medium sized biopharmaceutical enterprises has low resources and expertise to conduct preclinical studies and hence, they are increasingly outsourcing this tasks to the preclinical CROs, which in turn, boosts the growth of the preclinical CRO market across the globe. In this way the rising number of biopharmaceutical companies are positively impacting the market.
The rising burden of diseases is boosting the demand for various medical devices and diagnosis equipment that can provide fast and accurate results and re non-invasive. Therefore, rising investments by the medical device companies in the preclinical CRO services is another major factors that plays an exceptional role in the growth and development of the preclinical CRO market.
North America is the dominating preclinical CRO market that accounted for a market share of over 45% in 2020. The North America is characterized by the rapid growth of the CROs and biopharmaceutical companies. According to the Pharmaceutical Research and Manufacturers Association (PhRMA), US conducts more than half of the research & development activities in the pharmaceutical field and also holds intellectual rights of a significant amount of new medicines. The biopharmaceutical industry accounted for around 4% of the US GDP in 2015. Moreover, higher adoption rate of advanced technologies along with the adequate amount of funding is boosting the entire ecosystem of preclinical studies and drug development process in US.
Asia Pacific is well known for the presence of few top CROs in the regions like China, India, and South Korea. The favorable government policies to attract FDIs and rising government expenditure of building sophisticated healthcare infrastructure is favoring the growth of the preclinical CRO market in Asia Pacific. Moreover, the cost-efficiency of the CROs in this region is expected to drive the growth of the market during the forecast period.
Lack of adequate investments in the underdeveloped economies to develop and expand the preclinical CRO industry is anticipated to be a major challenge to the industry and this may hamper the market growth in the forthcoming years. Moreover, there is a lack of standardization as the CROs fails to comply with certain standards such as good laboratory practices and there work quality are questionable. This factor may hamper the market growth in the upcoming future.
Based on the service, the bioanalysis & DMPK studies segment is estimated to be the fastest-growing segment during the forecast period. Bioanalysis is provides quantitative insights regarding the preclinical studies and is used at each and every step of the new drug development process. Further, the DMPK facilitates the toxicology testing, which itself is a very large segment and hence the DMPK is expected to grow rapidly during the forecast period.
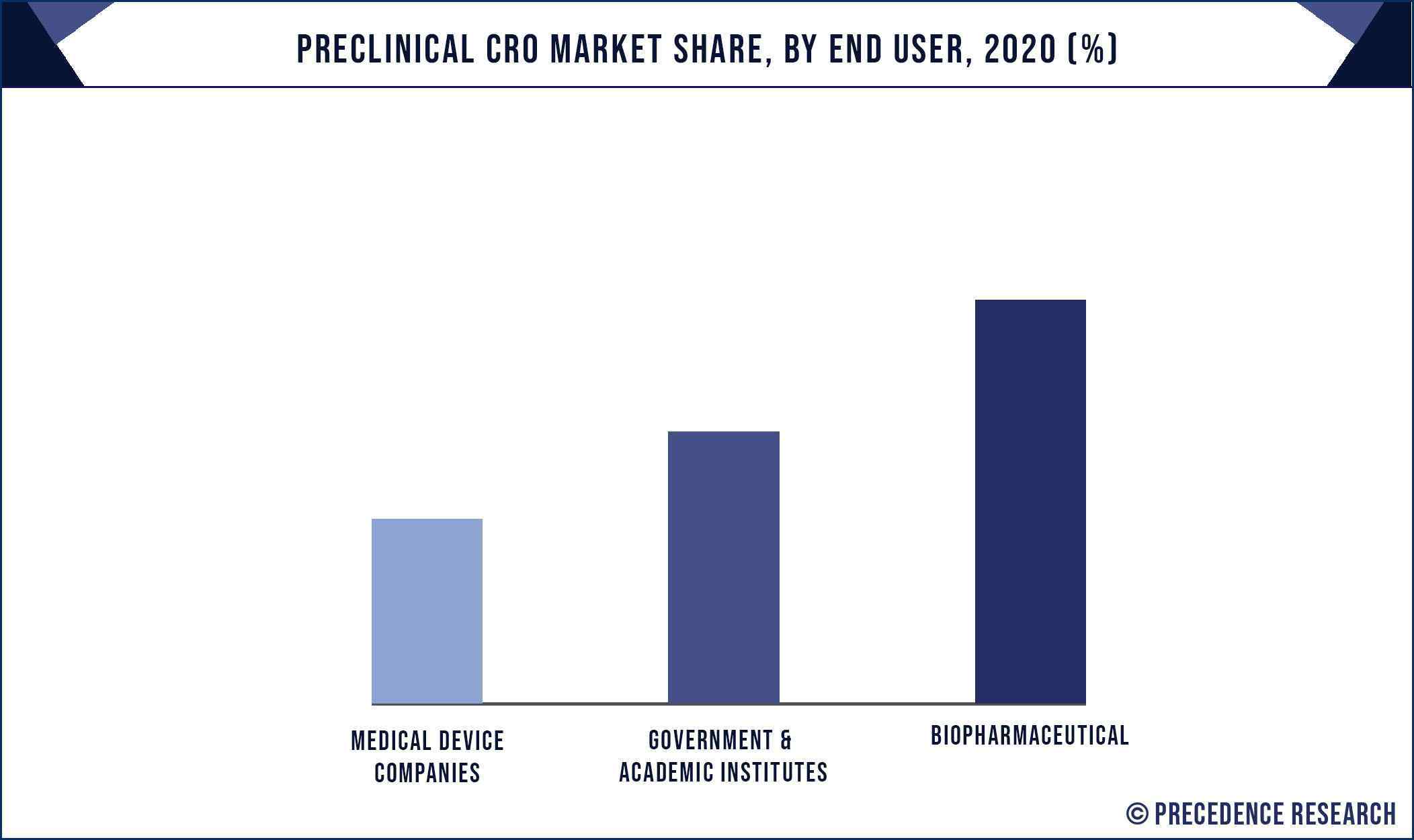
Based on the end use, the biopharmaceutical segment dominated the market, garnering a market share of around 80% in 2020. The rising government initiatives to fund and develop the biopharmaceutical industry resulted in a surging growth of this segment. Biopharmaceutical industry represents around 20% of the global pharmaceutical market and is expected to rise further in the foreseeable future, which in turn is expected to drive the demand for the preclinical CRO services.
Read Also: Healthcare Insurance Market Size is Estimated to be US$ 3,948.7 Bn by 2030
Key Players/Manufacturers
This report also provides detailed company profiles of the key market players. This research report also highlights the competitive landscape of the preclinical CRO market and ranks noticeable companies as per their occurrence in diverse regions across the globe and crucial developments initiated by them in the market space. This research study also tracks and evaluates competitive developments, such as collaborations, partnerships, and agreements, mergers and acquisitions; novel product introductions and developments, promotion strategies and Research and Development (R&D) activities in the marketplace. The competitive profiling of these players includes business and financial overview, gross margin, production, sales, and recent developments which can aid in assessing competition in the market.
Some of the prominent players in the global preclinical CRO market include:
- Wuxi AppTec
- Pharmaceutical Product Development
- Medpace, Inc.
- Charles River Laboratories International, Inc.
- PRA Health Science, Inc.
- PAREXEL
- Envigo
- Eurofins Scientific.
- Laboratory Corporation of America
- ICON Plc
Preclinical CRO Market Segments Covered
By Service
- Toxicology Testing
- Bioanalysis & DMPK Studies
- Chemistry
- Compound Management
- Safety Pharmacology
- Others
By End Use
- Medical Device Companies
- Biopharmaceutical
- Government & Academic Institutes
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Rest of the World
Research Objective
- To provide a comprehensive analysis of the preclinical CRO industry and its sub-segments in the global market, thereby providing a detailed structure of the industry
- To provide detailed insights into factors driving and restraining the growth of this global market
- To provide a distribution chain analysis/value chain for the this market
- To estimate the market size of the global preclinical CRO market where 2019 would be the historical period, 2020 shall be the base year, and 2020 to 2027 will be the forecast period for the study
- To provide strategic profiling of key companies (manufacturers and distributors) present across the globe, and comprehensively analyze their competitiveness/competitive landscape in this market
- To analyze the global market in four main geographies, namely, North America, Europe, Asia-Pacific, and the Rest of the World
- To provide country-wise market value analysis for various segments of the preclinical CRO
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Preclinical CRO Market
5.1. COVID-19 Landscape: Preclinical CRO Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Preclinical CRO Market, By Service
8.1. Preclinical CRO Market, by Service Type, 2021-2030
8.1.1. Toxicology Testing
8.1.1.1. Market Revenue and Forecast (2019-2030)
8.1.2. Bioanalysis & DMPK Studies
8.1.2.1. Market Revenue and Forecast (2019-2030)
8.1.3. Chemistry
8.1.3.1. Market Revenue and Forecast (2019-2030)
8.1.4. Compound Management
8.1.4.1. Market Revenue and Forecast (2019-2030)
8.1.5. Safety Pharmacology
8.1.5.1. Market Revenue and Forecast (2019-2030)
8.1.6. Others
8.1.6.1. Market Revenue and Forecast (2019-2030)
Chapter 9. Global Preclinical CRO Market, By End Use
9.1. Preclinical CRO Market, by End Use, 2021-2030
9.1.1. Medical Device Companies
9.1.1.1. Market Revenue and Forecast (2019-2030)
9.1.2. Biopharmaceutical
9.1.2.1. Market Revenue and Forecast (2019-2030)
9.1.3. Government & Academic Institutes
9.1.3.1. Market Revenue and Forecast (2019-2030)
Chapter 10. Global Preclinical CRO Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Service (2019-2030)
10.1.2. Market Revenue and Forecast, by End Use (2019-2030)
10.1.3. U.S.
10.1.3.1. Market Revenue and Forecast, by Service (2019-2030)
10.1.3.2. Market Revenue and Forecast, by End Use (2019-2030)
10.1.4. Rest of North America
10.1.4.1. Market Revenue and Forecast, by Service (2019-2030)
10.1.4.2. Market Revenue and Forecast, by End Use (2019-2030)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Service (2019-2030)
10.2.2. Market Revenue and Forecast, by End Use (2019-2030)
10.2.3. UK
10.2.3.1. Market Revenue and Forecast, by Service (2019-2030)
10.2.3.2. Market Revenue and Forecast, by End Use (2019-2030)
10.2.4. Germany
10.2.4.1. Market Revenue and Forecast, by Service (2019-2030)
10.2.4.2. Market Revenue and Forecast, by End Use (2019-2030)
10.2.5. France
10.2.5.1. Market Revenue and Forecast, by Service (2019-2030)
10.2.5.2. Market Revenue and Forecast, by End Use (2019-2030)
10.2.6. Rest of Europe
10.2.6.1. Market Revenue and Forecast, by Service (2019-2030)
10.2.6.2. Market Revenue and Forecast, by End Use (2019-2030)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Service (2019-2030)
10.3.2. Market Revenue and Forecast, by End Use (2019-2030)
10.3.3. India
10.3.3.1. Market Revenue and Forecast, by Service (2019-2030)
10.3.3.2. Market Revenue and Forecast, by End Use (2019-2030)
10.3.4. China
10.3.4.1. Market Revenue and Forecast, by Service (2019-2030)
10.3.4.2. Market Revenue and Forecast, by End Use (2019-2030)
10.3.5. Japan
10.3.5.1. Market Revenue and Forecast, by Service (2019-2030)
10.3.5.2. Market Revenue and Forecast, by End Use (2019-2030)
10.3.6. Rest of APAC
10.3.6.1. Market Revenue and Forecast, by Service (2019-2030)
10.3.6.2. Market Revenue and Forecast, by End Use (2019-2030)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Service (2019-2030)
10.4.2. Market Revenue and Forecast, by End Use (2019-2030)
10.4.3. GCC
10.4.3.1. Market Revenue and Forecast, by Service (2019-2030)
10.4.3.2. Market Revenue and Forecast, by End Use (2019-2030)
10.4.4. North Africa
10.4.4.1. Market Revenue and Forecast, by Service (2019-2030)
10.4.4.2. Market Revenue and Forecast, by End Use (2019-2030)
10.4.5. South Africa
10.4.5.1. Market Revenue and Forecast, by Service (2019-2030)
10.4.5.2. Market Revenue and Forecast, by End Use (2019-2030)
10.4.6. Rest of MEA
10.4.6.1. Market Revenue and Forecast, by Service (2019-2030)
10.4.6.2. Market Revenue and Forecast, by End Use (2019-2030)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Service (2019-2030)
10.5.2. Market Revenue and Forecast, by End Use (2019-2030)
10.5.3. Brazil
10.5.3.1. Market Revenue and Forecast, by Service (2019-2030)
10.5.3.2. Market Revenue and Forecast, by End Use (2019-2030)
10.5.4. Rest of LATAM
10.5.4.1. Market Revenue and Forecast, by Service (2019-2030)
10.5.4.2. Market Revenue and Forecast, by End Use (2019-2030)
Chapter 11. Company Profiles
11.1. Wuxi AppTec
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Pharmaceutical Product Development
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Medpace, Inc.
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. Charles River Laboratories International, Inc.
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. Recent Initiatives
11.5. PRA Health Science, Inc.
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. PAREXEL
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Envigo
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Eurofins Scientific.
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Laboratory Corporation of America
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. ICON Plc
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or the Asia Pacific.
Why Buy this Report?
The purpose of Precedence Research’s preclinical CRO market study is to provide stakeholders with a detailed picture of potential barriers and untapped opportunities. The report contains exclusive information to assist businesses in making informed decisions about how to maintain growth throughout the assessment period.
Buy Full Research Report (Single User License US$ 4500) @ https://www.precedenceresearch.com/checkout/1361
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com