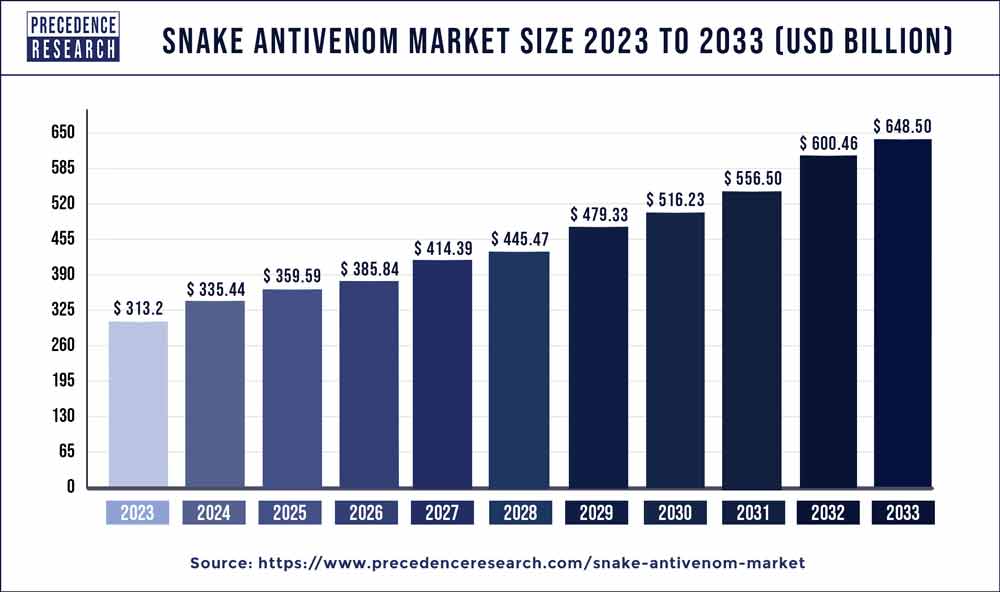
The global snake antivenom market size accounted for US$ 313.2 billion in 2023 and is projected to reach around USD 648.50 billion by 2033, growing at a CAGR of 7.60% from 2024 to 2033.
Key Takeaways
- North America contributed more than 42% of market share in 2023.
- Asia-Pacific is estimated to expand at the fastest CAGR between 2024 and 2033.
- By type, the polyvalent heterologous segment has held the largest market share of 59% in 2023.
- By type, the monovalent heterologous segment is anticipated to grow at a remarkable CAGR of 8.14% between 2024 and 2033.
- By application, the hospitals segment generated over 53% of market share in 2023.
- By application, the clinics segment is expected to expand at the fastest CAGR over the projected period.
Report Summary
The global snake antivenom market report provides a Point-by-Point and In-Depth analysis of global market size, regional and country-level market size, market share, segmentation market growth, competitive landscape, sales analysis, opportunities analysis, strategic market growth analysis, the impact of domestic and global market key players, value chain optimization, trade regulations, recent developments, product launches, area marketplace expanding, and technological innovations.
The study offers a comprehensive analysis on diverse features, including production capacities, demand, product developments, revenue generation, and sales in the snake antivenom market across the globe.
A comprehensive estimate on the snake antivenom market has been provided through an optimistic scenario as well as a conservative scenario, taking into account the sales of snake antivenom during the forecast period. Price point comparison by region with global average price is also considered in the study.
Download Access to a Free Copy of Our Latest Sample Report@ https://www.precedenceresearch.com/sample/3705
Snake Antivenom Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 7.60% |
| Global Market Size in 2023 | USD 313.2 Billion |
| Global Market Size by 2033 | USD 648.50 Billion |
| U.S. Market Size in 2023 | USD 92.08 Billion |
| U.S. Market Size by 2033 | USD 194.23 Billion |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Type and By Application |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Key Highlights:
Reports Coverage: It incorporates key market sections, key makers secured, the extent of items offered in the years considered, worldwide containerized snake antivenom market and study goals. Moreover, it contacts the division study gave in the report based on the sort of item and applications.
Market Outline: This area stresses the key investigations, market development rate, serious scene, market drivers, patterns, and issues notwithstanding the naturally visible pointers.
Market Production by Region: The report conveys information identified with import and fare, income, creation, and key players of every single local market contemplated are canvassed right now.
Also Read: Multi-factor Authentication Market Size to Grow USD 74.73 Bn By 2033
Market Players
The report includes the profiles of key snake antivenom market companies along with their SWOT analysis and market strategies. In addition, the report focuses on leading industry players with information such as company profiles, components and services offered, financial information, key development in past five years.
Recent Developments
- In August 2022, Bharat Serums and Vaccines Limited (BSV) collaborated with the Indian Institute of Science (IISc) to develop antivenom specifically tailored for snakebites in India, addressing the region’s unique challenges.
- In March 2022, Ophirex, Inc. achieved Fast Track Designation from the U.S. FDA for varespladib-methyl, a promising treatment for snakebite. This designation reflects the FDA’s acknowledgment of the drug’s potential benefits and the urgency to bring it to market efficiently.
- By April 2021, Rare Disease Therapeutics, Inc. expanded the FDA approval for Antivenom ANAVIP to treat North American Pit Viper Envenomation, showcasing the continuous development of effective snakebite therapies.
- In March 2021, SERB completed the acquisition of BTG International Inc., reinforcing its position as a global leader in critical care medicines and expanding its capacity to address healthcare challenges on a worldwide scale.
Snake Antivenom Market Companies
- CSL Limited
- Bharat Serums and Vaccines Limited (BSV)
- Rare Disease Therapeutics, Inc.
- Instituto Clodomiro Picado
- Vins Bioproducts Limited
- Inosan Biopharma
- Haffkine Bio-Pharmaceutical Corporation Limited
- MicroPharm Limited
- Instituto Butantan
- Serum Institute of India Pvt. Ltd.
- S. A. Altesa Lifesciences
- Flynn Pharma
- Instituto Nacional de Producción de Biológicos
- Vacsera
- Hetero Drugs Limited
Segments Covered in the Report
By Type
- Polyvalent Heterologous
- Monovalent Heterologous
By Application
- Hospitals
- Clinics
Regional Segmentation
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Southeast Asia and Rest of APAC)
- Latin America (Brazil and Rest of Latin America)
- Middle East and Africa (GCC, North Africa, South Africa, Rest of MEA)
Research Methodology
Secondary Research
It involves company databases such as Hoover’s: This assists us to recognize financial information, the structure of the market participants and industry’s competitive landscape.
The secondary research sources referred in the process are as follows:
- Governmental bodies, and organizations creating economic policies
- National and international social welfare institutions
- Company websites, financial reports and SEC filings, broker and investor reports
- Related patent and regulatory databases
- Statistical databases and market reports
- Corporate Presentations, news, press release, and specification sheet of Manufacturers
Primary Research
Primary research includes face-to-face interviews, online surveys, and telephonic interviews.
- Means of primary research: Email interactions, telephonic discussions and Questionnaire-based research etc.
- In order to validate our research findings and analysis, we conduct primary interviews of key industry participants. Insights from primary respondents help in validating the secondary research findings. It also develops Research Team’s expertise and market understanding.
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology (Premium Insights)
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Snake Antivenom Market
5.1. COVID-19 Landscape: Snake Antivenom Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Snake Antivenom Market, By Type
8.1. Snake Antivenom Market Revenue and Volume, by Type, 2024-2033
8.1.1. Polyvalent Heterologous
8.1.1.1. Market Revenue and Volume Forecast (2021-2033)
8.1.2. Monovalent Heterologous
8.1.2.1. Market Revenue and Volume Forecast (2021-2033)
Chapter 9. Global Snake Antivenom Market, By Application
9.1. Snake Antivenom Market Revenue and Volume, by Application, 2024-2033
9.1.1. Hospitals
9.1.1.1. Market Revenue and Volume Forecast (2021-2033)
9.1.2. Clinics
9.1.2.1. Market Revenue and Volume Forecast (2021-2033)
Chapter 10. Global Snake Antivenom Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.1.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.1.3. U.S.
10.1.3.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.1.3.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.1.4. Rest of North America
10.1.4.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.1.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.2. Europe
10.2.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.2.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.2.3. UK
10.2.3.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.2.3.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.2.4. Germany
10.2.4.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.2.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.2.5. France
10.2.5.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.2.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.2.6. Rest of Europe
10.2.6.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.2.6.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.3. APAC
10.3.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.3.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.3.3. India
10.3.3.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.3.3.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.3.4. China
10.3.4.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.3.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.3.5. Japan
10.3.5.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.3.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.3.6. Rest of APAC
10.3.6.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.3.6.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.4. MEA
10.4.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.4.3. GCC
10.4.3.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.4.3.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.4.4. North Africa
10.4.4.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.4.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.4.5. South Africa
10.4.5.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.4.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.4.6. Rest of MEA
10.4.6.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.4.6.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.5. Latin America
10.5.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.5.3. Brazil
10.5.3.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.5.3.2. Market Revenue and Volume Forecast, by Application (2021-2033)
10.5.4. Rest of LATAM
10.5.4.1. Market Revenue and Volume Forecast, by Type (2021-2033)
10.5.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
Chapter 11. Company Profiles
11.1. CSL Limited
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Bharat Serums and Vaccines Limited (BSV)
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. Rare Disease Therapeutics, Inc.
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. Instituto Clodomiro Picado
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. Recent Initiatives
11.5. Vins Bioproducts Limited
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Inosan Biopharma
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Haffkine Bio-Pharmaceutical Corporation Limited
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. MicroPharm Limited
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Instituto Butantan
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. Serum Institute of India Pvt. Ltd.
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or the Asia Pacific.
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com