Key Takeaways
- By product, the reagents segment dominated the market with the largest share of 67% in 2023.
- By test location, the point-of-care segment dominated the market in 2023.
- By technology, the Immunoassay segment is expected to dominate the market during the forecast period.
- By application, the infectious diseases segment led the market with the largest share in 2023.
- By application, the oncology segment is expected witness the fastest rate of growth during the forecast period of 2024-2033.
- By end user, the hospital segment held the dominating share of the market in 2023.
Introduction:
The U.S. in vitro diagnostics (IVD) market represents a crucial sector within the healthcare industry, playing a pivotal role in disease diagnosis, monitoring, and management. IVD encompasses a wide range of tests conducted on biological samples such as blood, urine, and tissues outside the human body. These tests provide vital information to healthcare professionals for making accurate and timely medical decisions. With advancements in technology and increasing demand for personalized healthcare solutions, the U.S. IVD market continues to evolve, offering innovative diagnostic tools and solutions to address diverse medical needs.
Get a Sample: https://www.precedenceresearch.com/sample/3712
Growth Factors:
Several factors contribute to the growth of the U.S. IVD market. Technological advancements, such as the integration of artificial intelligence (AI) and molecular diagnostics, have enhanced the accuracy and efficiency of diagnostic tests, driving market expansion. Additionally, the rising prevalence of chronic and infectious diseases, coupled with the growing geriatric population, increases the demand for diagnostic procedures, fueling market growth. Moreover, the shift towards preventive healthcare measures and the emphasis on early disease detection further stimulate market expansion, creating opportunities for innovation and investment in the IVD sector.
U.S. In Vitro Diagnostics Market Scope
| Report Coverage | Details |
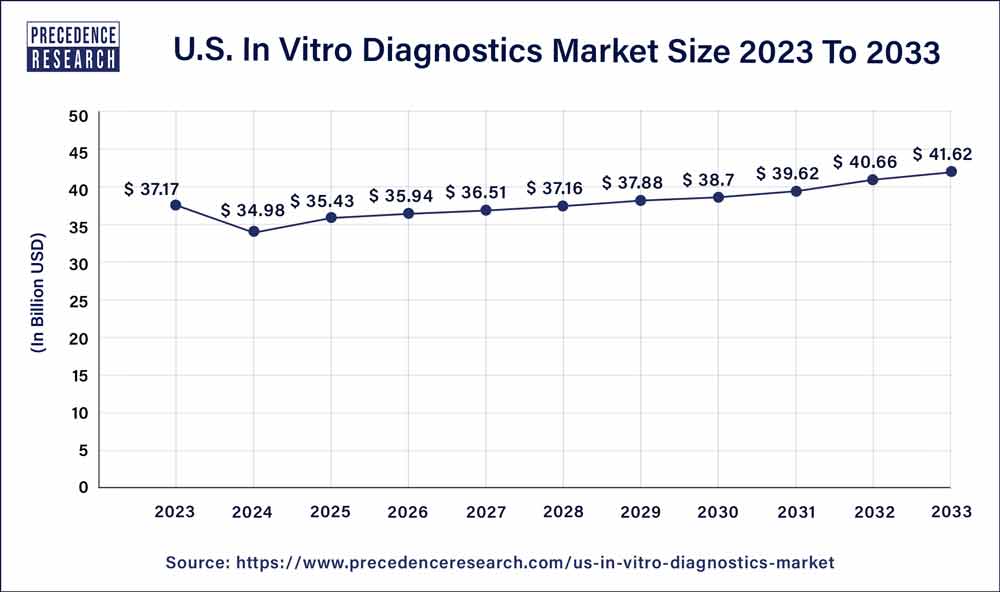
| U.S. Market Size in 2023 | USD 37.17 Billion |
| U.S. Market Size by 2033 | USD 41.62 Billion |
| Growth Rate from 2024 to 2033 | CAGR of 2% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Product, By Test Location, By Technology, By Application, and By End User |
U.S. In Vitro Diagnostics Market Dynamics
Drivers:
Several key drivers propel the growth of the U.S. IVD market. One significant driver is the increasing adoption of point-of-care testing (POCT) devices, which offer rapid results and convenience, particularly in emergency and remote healthcare settings. Additionally, the rising demand for companion diagnostics, especially in oncology and personalized medicine, drives market growth. Furthermore, favorable regulatory initiatives aimed at streamlining the approval process for new diagnostic tests and technologies accelerate market expansion. Moreover, the growing focus on healthcare digitization and telemedicine practices amplifies the demand for connected diagnostic devices, fostering market growth opportunities.
Restraints:
Despite its growth potential, the U.S. IVD market faces certain challenges and restraints. One notable restraint is the stringent regulatory framework governing the approval and commercialization of diagnostic tests, which can lead to delays and increased costs for market players. Additionally, reimbursement complexities and pricing pressures pose challenges for manufacturers, especially in the highly competitive market landscape. Moreover, concerns regarding data privacy and security in the era of digital diagnostics raise apprehensions among patients and healthcare providers, potentially hindering market growth.
Opportunities:
Despite the challenges, the U.S. IVD market presents significant opportunities for innovation and market expansion. The growing demand for rapid diagnostic solutions, especially in the wake of global health crises such as the COVID-19 pandemic, creates opportunities for manufacturers to develop novel testing platforms and solutions. Moreover, the increasing focus on precision medicine and personalized healthcare drives demand for advanced molecular diagnostics and biomarker-based tests, offering avenues for market growth. Furthermore, strategic collaborations between industry players and healthcare stakeholders, along with investments in research and development, can unlock new opportunities and drive the evolution of the U.S. IVD market.
Read Also: Road Rollers Market Size to Rise USD 6.97 Billion By 2033
Recent Developments
- In November 2023, to create molecular tests for decentralized in vitro diagnostic (IVD) applications, Illumina, a leader in DNA sequencing and array-based technologies, and Veracyte, a top genomic diagnostics business, have partnered for several years. The partnership is centered on using Illumina’s NextSeq 550Dx equipment to develop Veracyte’s Percepta Nasal Swab test and Prosigna Breast Cancer Assay.
- In March 2023, Eli Lilly & Company and Roche announced their partnership to promote the development of Roche’s Elecsys Amyloid Plasma Panel (EAPP). A novel blood test called the EAPP promises to help diagnose Alzheimer’s disease early.
- In April 2023, to enhance health outcomes worldwide, Oxford Nanopore Technologies and bioMérieux SA, a pioneer in the in vitro diagnostics industry, announced that they have joined forces to investigate specific prospects to introduce nanopore sequencing to the infectious disease diagnostics market.
U.S. In Vitro Diagnostics Market Companies
- Alere, Inc.
- Beckman Coulter
- BD
- Bio-Rad laboratories
- Danaher
- Abbott Laboratories
- Thermo Fisher Scientific Inc.
- bioMérieux, Inc
- Quest Diagnostics
- Illumina, Inc.
Segments Covered in the Report
By Product
- Reagents
- Instruments
- Services
By Test Location
- Point of Care
- Home Care
- Others
By Technology
- Immunoassay
- Instruments
- Reagents
- Services
- Hematology
- Instruments
- Reagents
- Services
- Clinical Chemistry
- Instruments
- Reagents
- Services
- Molecular Diagnostics
- Instruments
- Reagents
- Services
- Coagulation
- Instruments
- Reagents
- Services
- Microbiology
- Instruments
- Reagents
- Services
- Others
- Instruments
- Reagents
- Services
By Application
- Diabetes
- Cardiology
- Nephrology
- Infectious Disease
- Oncology
- Drug Testing
- Autoimmune Diseases
- Others
By End User
- Standalone Laboratories
- Hospitals
- Academic & Medical Schools
- Point-of-Care
- Others
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology (Premium Insights)
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on U.S. In Vitro Diagnostics Market
5.1. COVID-19 Landscape: U.S. In Vitro Diagnostics Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global U.S. In Vitro Diagnostics Market, By Product
8.1. U.S. In Vitro Diagnostics Market Revenue and Volume, by Product, 2023-2032
8.1.1. Reagents
8.1.1.1. Market Revenue and Volume Forecast (2020-2032)
8.1.2. Instruments
8.1.2.1. Market Revenue and Volume Forecast (2020-2032)
8.1.3. Services
8.1.3.1. Market Revenue and Volume Forecast (2020-2032)
Chapter 9. Global U.S. In Vitro Diagnostics Market, By Test Location
9.1. U.S. In Vitro Diagnostics Market Revenue and Volume, by Test Location, 2023-2032
9.1.1. Point of Care
9.1.1.1. Market Revenue and Volume Forecast (2020-2032)
9.1.2. Home Care
9.1.2.1. Market Revenue and Volume Forecast (2020-2032)
9.1.3. Others
9.1.3.1. Market Revenue and Volume Forecast (2020-2032)
Chapter 10. Global U.S. In Vitro Diagnostics Market, By Technology
10.1. U.S. In Vitro Diagnostics Market Revenue and Volume, by Technology, 2023-2032
10.1.1. Immunoassay
10.1.1.1. Market Revenue and Volume Forecast (2020-2032)
10.1.2. Hematology
10.1.2.1. Market Revenue and Volume Forecast (2020-2032)
10.1.3. Clinical Chemistry
10.1.3.1. Market Revenue and Volume Forecast (2020-2032)
10.1.4. Molecular Diagnostics
10.1.4.1. Market Revenue and Volume Forecast (2020-2032)
10.1.5. Coagulation
10.1.5.1. Market Revenue and Volume Forecast (2020-2032)
10.1.6. Microbiology
10.1.6.1. Market Revenue and Volume Forecast (2020-2032)
10.1.7. Others
10.1.7.1. Market Revenue and Volume Forecast (2020-2032)
Chapter 11. Global U.S. In Vitro Diagnostics Market, By Application
11.1. U.S. In Vitro Diagnostics Market Revenue and Volume, by Application, 2023-2032
11.1.1. Diabetes
11.1.1.1. Market Revenue and Volume Forecast (2020-2032)
11.1.2. Cardiology
11.1.2.1. Market Revenue and Volume Forecast (2020-2032)
11.1.3. Nephrology
11.1.3.1. Market Revenue and Volume Forecast (2020-2032)
11.1.4. Infectious Disease
11.1.4.1. Market Revenue and Volume Forecast (2020-2032)
11.1.5. Oncology
11.1.5.1. Market Revenue and Volume Forecast (2020-2032)
11.1.6. Drug Testing
11.1.6.1. Market Revenue and Volume Forecast (2020-2032)
11.1.7. Autoimmune Diseases
11.1.7.1. Market Revenue and Volume Forecast (2020-2032)
11.1.8. Others
11.1.8.1. Market Revenue and Volume Forecast (2020-2032)
Chapter 12. Global U.S. In Vitro Diagnostics Market, By End User
12.1. U.S. In Vitro Diagnostics Market Revenue and Volume, by End User, 2023-2032
12.1.1. Standalone Laboratories
12.1.1.1. Market Revenue and Volume Forecast (2020-2032)
12.1.2. Hospitals
12.1.2.1. Market Revenue and Volume Forecast (2020-2032)
12.1.3. Academic & Medical Schools
12.1.3.1. Market Revenue and Volume Forecast (2020-2032)
12.1.4. Point-of-Care
12.1.4.1. Market Revenue and Volume Forecast (2020-2032)
12.1.5. Others
12.1.5.1. Market Revenue and Volume Forecast (2020-2032)
Chapter 13. Global U.S. In Vitro Diagnostics Market, Regional Estimates and Trend Forecast
13.1. North America
13.1.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.1.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.1.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.1.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.1.5. Market Revenue and Volume Forecast, by End User (2020-2032)
13.1.6. U.S.
13.1.6.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.1.6.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.1.6.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.1.6.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.1.6.5. Market Revenue and Volume Forecast, by End User (2020-2032)
13.1.7. Rest of North America
13.1.7.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.1.7.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.1.7.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.1.7.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.1.7.5. Market Revenue and Volume Forecast, by End User (2020-2032)
13.2. Europe
13.2.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.2.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.2.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.2.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.2.5. Market Revenue and Volume Forecast, by End User (2020-2032)
13.2.6. UK
13.2.6.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.2.6.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.2.6.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.2.7. Market Revenue and Volume Forecast, by Application (2020-2032)
13.2.8. Market Revenue and Volume Forecast, by End User (2020-2032)
13.2.9. Germany
13.2.9.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.2.9.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.2.9.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.2.10. Market Revenue and Volume Forecast, by Application (2020-2032)
13.2.11. Market Revenue and Volume Forecast, by End User (2020-2032)
13.2.12. France
13.2.12.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.2.12.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.2.12.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.2.12.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.2.13. Market Revenue and Volume Forecast, by End User (2020-2032)
13.2.14. Rest of Europe
13.2.14.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.2.14.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.2.14.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.2.14.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.2.15. Market Revenue and Volume Forecast, by End User (2020-2032)
13.3. APAC
13.3.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.3.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.3.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.3.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.3.5. Market Revenue and Volume Forecast, by End User (2020-2032)
13.3.6. India
13.3.6.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.3.6.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.3.6.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.3.6.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.3.7. Market Revenue and Volume Forecast, by End User (2020-2032)
13.3.8. China
13.3.8.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.3.8.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.3.8.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.3.8.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.3.9. Market Revenue and Volume Forecast, by End User (2020-2032)
13.3.10. Japan
13.3.10.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.3.10.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.3.10.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.3.10.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.3.10.5. Market Revenue and Volume Forecast, by End User (2020-2032)
13.3.11. Rest of APAC
13.3.11.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.3.11.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.3.11.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.3.11.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.3.11.5. Market Revenue and Volume Forecast, by End User (2020-2032)
13.4. MEA
13.4.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.4.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.4.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.4.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.4.5. Market Revenue and Volume Forecast, by End User (2020-2032)
13.4.6. GCC
13.4.6.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.4.6.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.4.6.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.4.6.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.4.7. Market Revenue and Volume Forecast, by End User (2020-2032)
13.4.8. North Africa
13.4.8.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.4.8.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.4.8.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.4.8.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.4.9. Market Revenue and Volume Forecast, by End User (2020-2032)
13.4.10. South Africa
13.4.10.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.4.10.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.4.10.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.4.10.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.4.10.5. Market Revenue and Volume Forecast, by End User (2020-2032)
13.4.11. Rest of MEA
13.4.11.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.4.11.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.4.11.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.4.11.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.4.11.5. Market Revenue and Volume Forecast, by End User (2020-2032)
13.5. Latin America
13.5.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.5.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.5.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.5.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.5.5. Market Revenue and Volume Forecast, by End User (2020-2032)
13.5.6. Brazil
13.5.6.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.5.6.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.5.6.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.5.6.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.5.7. Market Revenue and Volume Forecast, by End User (2020-2032)
13.5.8. Rest of LATAM
13.5.8.1. Market Revenue and Volume Forecast, by Product (2020-2032)
13.5.8.2. Market Revenue and Volume Forecast, by Test Location (2020-2032)
13.5.8.3. Market Revenue and Volume Forecast, by Technology (2020-2032)
13.5.8.4. Market Revenue and Volume Forecast, by Application (2020-2032)
13.5.8.5. Market Revenue and Volume Forecast, by End User (2020-2032)
Chapter 14. Company Profiles
14.1. Alere, Inc.
14.1.1. Company Overview
14.1.2. Product Offerings
14.1.3. Financial Performance
14.1.4. Recent Initiatives
14.2. Beckman Coulter
14.2.1. Company Overview
14.2.2. Product Offerings
14.2.3. Financial Performance
14.2.4. Recent Initiatives
14.3. BD
14.3.1. Company Overview
14.3.2. Product Offerings
14.3.3. Financial Performance
14.3.4. Recent Initiatives
14.4. Bio-Rad laboratories
14.4.1. Company Overview
14.4.2. Product Offerings
14.4.3. Financial Performance
14.4.4. Recent Initiatives
14.5. Danaher
14.5.1. Company Overview
14.5.2. Product Offerings
14.5.3. Financial Performance
14.5.4. Recent Initiatives
14.6. Abbott Laboratories
14.6.1. Company Overview
14.6.2. Product Offerings
14.6.3. Financial Performance
14.6.4. Recent Initiatives
14.7. Thermo Fisher Scientific Inc.
14.7.1. Company Overview
14.7.2. Product Offerings
14.7.3. Financial Performance
14.7.4. Recent Initiatives
14.8. bioMérieux, Inc
14.8.1. Company Overview
14.8.2. Product Offerings
14.8.3. Financial Performance
14.8.4. Recent Initiatives
14.9. Quest Diagnostics
14.9.1. Company Overview
14.9.2. Product Offerings
14.9.3. Financial Performance
14.9.4. Recent Initiatives
14.10. Illumina, Inc.
14.10.1. Company Overview
14.10.2. Product Offerings
14.10.3. Financial Performance
14.10.4. Recent Initiatives
Chapter 15. Research Methodology
15.1. Primary Research
15.2. Secondary Research
15.3. Assumptions
Chapter 16. Appendix
16.1. About Us
16.2. Glossary of Terms
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
