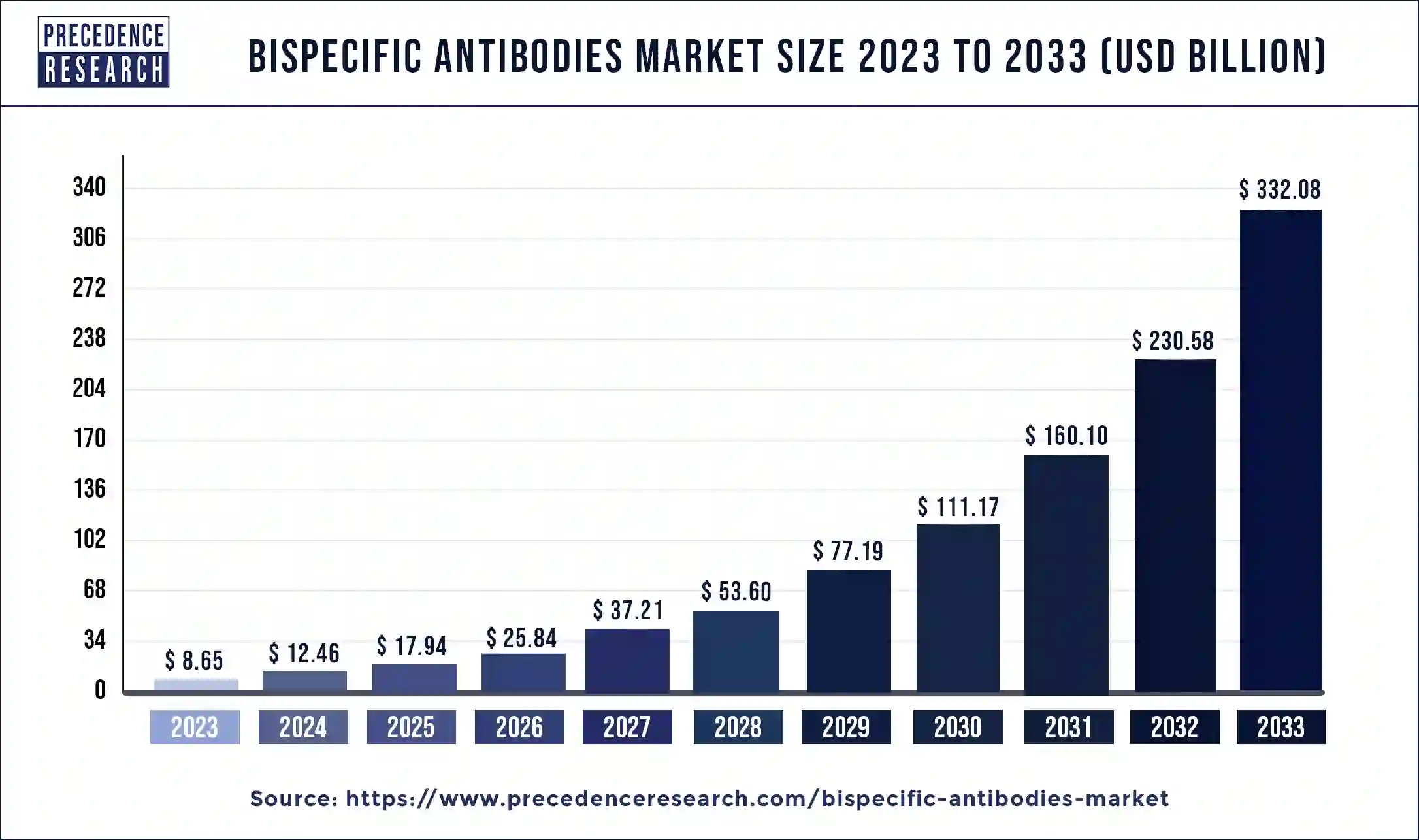
The global bispecific antibodies market size surpassed USD 8.65 billion in 2023 and is anticipated to grow around USD 332.08 billion by 2033, expanding at a CAGR of 44.02% from 2024 to 2033.
Key Points
- North America has contributed more than 88% of the market share in 2023.
- Europe is estimated to expand at the fastest CAGR between 2024 and 2033.
- By indication, the cancer segment held the highest market share in 2023.
- By indication, the inflammatory and autoimmune disorders segment is anticipated to witness rapid growth at a significant CAGR during the projected period.
- By distribution channel, the hospital pharmacies segment has held the biggest market share in 2023.
- By distribution channel, the retail pharmacies segment is anticipated to witness significant growth over the projected period.
The bispecific antibodies market has been witnessing significant growth in recent years, driven by advancements in biotechnology and immunotherapy. Bispecific antibodies are engineered proteins designed to simultaneously target two different antigens, which can lead to enhanced therapeutic effects compared to traditional monoclonal antibodies. These innovative molecules hold immense potential for treating various diseases, including cancer, autoimmune disorders, and infectious diseases. As the understanding of bispecific antibody technology improves and more therapeutic applications emerge, the market is poised for substantial expansion in the coming years.
Get a Sample: https://www.precedenceresearch.com/sample/3977
Growth Factors:
Several factors contribute to the growth of the bispecific antibodies market. One of the primary drivers is the increasing prevalence of cancer and other chronic diseases globally. Bispecific antibodies offer a promising approach for cancer therapy by targeting multiple pathways simultaneously, thereby potentially overcoming tumor heterogeneity and treatment resistance. Moreover, advancements in antibody engineering techniques have facilitated the development of bispecific antibodies with improved efficacy and reduced immunogenicity, further driving market growth.
Additionally, the growing investments in research and development (R&D) by pharmaceutical and biotechnology companies are fueling innovation in the field of bispecific antibodies. Collaborations between academic institutions, research organizations, and industry players are leading to the discovery of novel bispecific antibody candidates and the exploration of new therapeutic indications. Furthermore, the expedited regulatory pathways for biologics and the increasing adoption of personalized medicine are creating favorable conditions for the commercialization of bispecific antibody therapies, contributing to market expansion.
Region Insights:
The bispecific antibodies market exhibits significant regional variations, influenced by factors such as healthcare infrastructure, regulatory environment, and prevalence of target diseases. North America dominates the market, owing to the presence of key biotechnology and pharmaceutical companies, robust R&D activities, and favorable reimbursement policies. The region also benefits from a high prevalence of cancer and autoimmune disorders, driving the demand for innovative therapeutics like bispecific antibodies.
Europe is another lucrative market for bispecific antibodies, characterized by a strong biopharmaceutical industry and supportive regulatory frameworks. The region’s emphasis on translational research and clinical development contributes to the adoption of novel therapeutic modalities, including bispecific antibodies. Moreover, Asia-Pacific is emerging as a promising market, propelled by increasing investments in healthcare infrastructure, rising disposable incomes, and a growing patient population with unmet medical needs.
Bispecific Antibodies Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 44.02% |
| Global Market Size in 2023 | USD 8.65 Billion |
| Global Market Size by 2033 | USD 332.08 Billion |
| U.S. Market Size in 2023 | USD 5.33 Billion |
| U.S. Market Size by 2033 | USD 206.02 Billion |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Indication and By Distribution Channel |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Bispecific Antibodies Dynamics
Drivers:
Several drivers are propelling the growth of the bispecific antibodies market. The increasing incidence of cancer and autoimmune diseases worldwide necessitates the development of more effective and targeted therapies. Bispecific antibodies offer the potential to address the limitations of traditional monoclonal antibodies by simultaneously targeting multiple disease pathways, enhancing therapeutic efficacy.
Moreover, advancements in antibody engineering technologies, such as bispecific T-cell engagers (BiTEs) and antibody-drug conjugates (ADCs), enable precise targeting of tumor cells while minimizing off-target effects. This targeted approach not only improves patient outcomes but also reduces the likelihood of adverse events, making bispecific antibodies an attractive option for cancer treatment.
Furthermore, the expanding applications of bispecific antibodies beyond oncology, such as infectious diseases and inflammatory disorders, are driving market growth. Researchers are exploring the therapeutic potential of bispecific antibodies in various therapeutic areas, opening up new avenues for drug development and commercialization.
Opportunities:
The bispecific antibodies market presents numerous opportunities for industry players and stakeholders. One key opportunity lies in the development of bispecific antibody combinations or multi-specific antibodies targeting multiple disease pathways simultaneously. By harnessing the synergistic effects of different antigen targets, these innovative therapeutics have the potential to revolutionize disease treatment and improve patient outcomes.
Moreover, the expansion of bispecific antibody platforms to incorporate novel technologies, such as gene editing and modular design approaches, offers exciting prospects for therapeutic innovation. These next-generation bispecific antibodies hold promise for overcoming existing challenges, such as limited stability, short half-life, and immunogenicity, thereby expanding the therapeutic utility of bispecific antibodies across various disease indications.
Additionally, strategic partnerships and collaborations between pharmaceutical companies, biotechnology firms, and academic institutions present opportunities for sharing expertise, resources, and intellectual property rights. Such collaborations can accelerate the development and commercialization of bispecific antibody therapies, enabling faster access to novel treatments for patients worldwide.
Challenges:
Despite the promising growth prospects, the bispecific antibodies market faces several challenges that warrant attention. One significant challenge is the complexity of manufacturing bispecific antibodies at a large scale while maintaining product quality and consistency. The production process often involves sophisticated techniques, such as cell line engineering and purification, which can result in high production costs and manufacturing challenges.
Furthermore, immunogenicity and safety concerns associated with bispecific antibodies remain important considerations in drug development. The potential for off-target effects, cytokine release syndrome, and immune-related adverse events necessitates thorough preclinical and clinical evaluation to ensure the safety and efficacy of bispecific antibody therapies.
Moreover, regulatory requirements for bispecific antibodies may vary across different regions, posing challenges for global drug development and commercialization. Harmonizing regulatory standards and establishing clear guidelines for bispecific antibody approval can streamline the regulatory process and facilitate market access for innovative therapies.
Read Also: Vitamin K Market Size to Hit USD 2,126.08 Million by 2033
Recent Developments
- In January 2023, the FDA granted approval to Genentech for bispecific antibodies intended for lymphoma treatment.
- In July 2022, Janssen Pharmaceutical announced the receipt of conditional marketing authorization for Teclistamab in multiple myeloma patients from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP). Additionally, the company has submitted a Biologics License Application (BLA) to the US FDA seeking clearance for its innovative medications.
Bispecific Antibodies Market Companies
- Adimab, Innovent Biologics, Inc (U.S.)
- Affimed GmbH (China)
- Amgen Inc (Germany)
- AstraZeneca (U.K.)
- Xencor (U.S.)
- Sanofi (France)
- F. Hoffmann-La Roche Ltd (Switzerland)
- Regeneron Pharmaceuticals Inc (U.S.)
- Eli Lilly (U.S.)
- Pieris Pharmaceuticals, Inc (U.S.)
- Mereo BioPharma Group plc (U.K)
- Sobi, TG Therapeutics Inc (Swden)
- Merus (Netherlands)
- MacroGenics, Inc (U.S.)
- Genmab A/S (Denmark)
- Emergent BioSolutions Inc (U.S.)
- Alteogen (South Korea)
- Astellas Pharma Inc (Japan)
- Novartis AG (Switzerland)
- CELGENE CORPORATION (U.S.)
Segments Covered in the Report
By Indication
- Cancer
- Inflammatory & Autoimmune Disorder
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Drugstores
- Online Pharmacies
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/