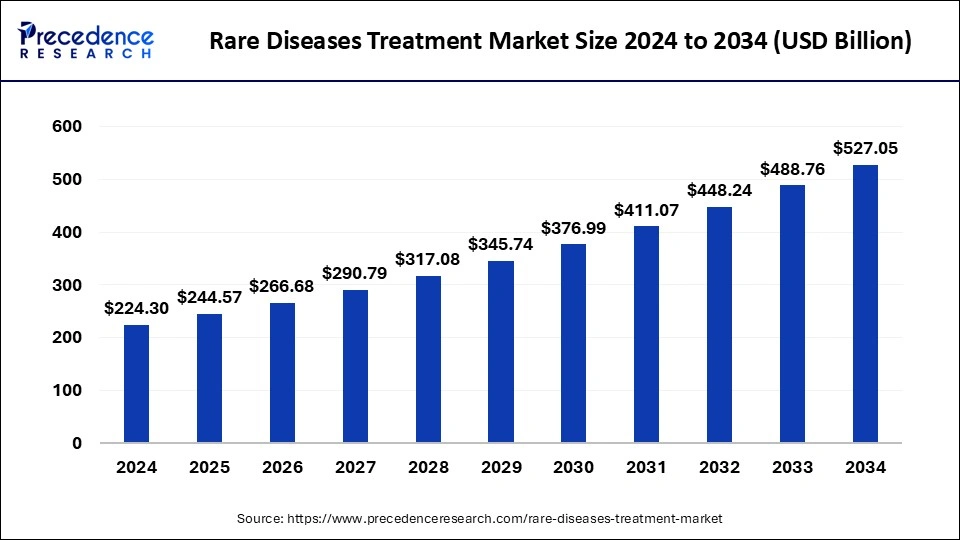
The global rare diseases treatment market size is expected to increase USD 488.76 billion by 2033 from USD 205.70 billion in 2023, with a CAGR of 9.04% between 2024 and 2033.
Key Points
- North America dominated the market with the biggest revenue share of 49% in 2023.
- Asia Pacific is expected to grow at the highest CAGR in the market during the forecast period.
- By drug type, the biologics segment has contributed more than 82% of revenue share in 2023.
- By drug type, the non-biologic segment is expected to grow rapidly in the market during the forecast period.
- By therapeutic area, the cancer segment has held a major revenue share of 50% in 2023.
- By therapeutic area, the blood-related disorder segment is expected to grow at a significant CAGR in the market during the forecast period.
- By route of administration, the injectable segment dominated the market in 2023.
- By route of administration, the oral segment is expected to grow at the highest CAGR in the market during the forecast period.
The rare diseases treatment market is characterized by its focus on addressing medical conditions that affect a small percentage of the population. These diseases often have complex etiologies and manifestations, making treatment challenging. Despite their rarity, the collective burden of rare diseases is significant, driving the need for effective therapies. The market encompasses various treatment modalities, including pharmacological interventions, gene therapies, and supportive care.
Get a Sample: https://www.precedenceresearch.com/sample/4410
Growth Factors:
Several factors contribute to the growth of the rare diseases treatment market. Advances in genetic research have facilitated the identification of underlying causes of many rare diseases, paving the way for targeted therapies. Additionally, regulatory incentives such as orphan drug designations and expedited approval pathways have encouraged pharmaceutical companies to invest in rare disease research and development. Rising awareness among healthcare professionals and patients about these conditions has also fueled demand for innovative treatments.
Region Insights:
The prevalence of rare diseases and the availability of treatment options vary across regions. Developed countries with robust healthcare infrastructure tend to have better diagnostic capabilities and access to specialized care, driving market growth. However, emerging economies are increasingly recognizing the importance of addressing rare diseases and are investing in healthcare infrastructure and research initiatives. Collaborative efforts between governments, academia, and industry stakeholders are crucial for advancing rare disease treatment globally.
Rare Diseases Treatment Market Scope
| Report Coverage | Details |
| Rare Diseases Treatment Market Size in 2023 | USD 205.70 Billion |
| Rare Diseases Treatment Market Size in 2024 | USD 224.30 Billion |
| Rare Diseases Treatment Market Size by 2033 | USD 488.76 Billion |
| Rare Diseases Treatment Market Growth Rate | CAGR of 9.04% from 2024 to 2033 |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Drug Type, Therapeutic Area, Patient, Route of Administration, Distribution Channel, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Rare Diseases Treatment Market Dynamics
Drivers:
Several drivers propel the growth of the rare diseases treatment market. The growing prevalence of genetic disorders, coupled with advancements in diagnostic technologies, has increased the identification of rare diseases, creating a larger patient pool. Moreover, increasing investments in precision medicine and personalized therapies have spurred the development of tailored treatments for specific rare conditions. Additionally, rising patient advocacy and support groups have amplified the voice of rare disease communities, driving awareness and research funding.
Opportunities:
The rare diseases treatment market presents numerous opportunities for innovation and collaboration. Advances in gene editing technologies such as CRISPR-Cas9 hold promise for correcting genetic defects underlying certain rare diseases. Furthermore, expanding access to healthcare in underserved regions and improving diagnostic capabilities can enhance early detection and intervention. Collaboration between academia, industry, and regulatory agencies can expedite the development and approval of novel therapies for rare diseases.
Challenges:
Despite significant progress, the rare diseases treatment market faces several challenges. Limited understanding of the molecular mechanisms underlying many rare diseases hinders the development of targeted therapies. Additionally, the high cost of research and development, coupled with the small patient populations, makes it economically challenging for pharmaceutical companies to invest in rare disease treatments. Access to affordable therapies remains a concern, particularly in resource-constrained settings, highlighting the need for sustainable pricing models and reimbursement strategies. Moreover, regulatory hurdles and lengthy approval processes can impede the timely delivery of innovative treatments to patients in need. Addressing these challenges requires concerted efforts from all stakeholders to ensure equitable access to effective rare disease therapies.
Read Also: Mutual Fund Assets Market Size to Worth USD 1,146.27 Bn by 2033
Rare Diseases Treatment Market Recent Developments
- In May 2024, After a 2023 approval from the FDA, Krystal Biotech collected more than $95 million from its launch of Vyjuvek, the first treatment for the rare skin disease dystrophic epidermolysis bullosa (DEB).
- In February 2024, Zydus Lifesciences announced the launch of its first new drug in the United States by early 2026, looking to tap into the multi-billion dollar market for treating a type of liver disease, Managing Director Sharvil Patel told Reuters.
- In February 2024, Florida State University launched the Institute for Pediatric Rare Diseases, an institute dedicated to advancing research and developing treatments for unusual childhood diseases, filling a critical gap in the healthcare industry. The institute is made possible by $1 million in funding from the Florida Legislature.
Rare Diseases Treatment Market Companies
- F. Hoffmann-La Roche Ltd.
- Pfizer, Inc.
- PTC Therapeutics
- AstraZeneca
- Novartis AG
- Takeda Pharmaceutical Company
- Bayer AG
- AbbVie Inc.
- Merck & Co. Inc.
- Bristol Myers Squibb
Segment Covered in the Report
By Drug Type
- Biologics
- Non-biologics
By Therapeutic Area
- Cancer
- Blood-Related Disorder
- Central Nervous System
- Respiratory Disorders
- Musculoskeletal Disorders
- Cardiovascular Disorder
- Other Therapeutic Areas
By Patient
- Adult
- Pediatric
By Route of Administration
- Oral
- Injectable
- Others
By Distribution Channel
- Hospital Pharmacy
- Specialty Pharmacy
- Online Pharmacy
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/