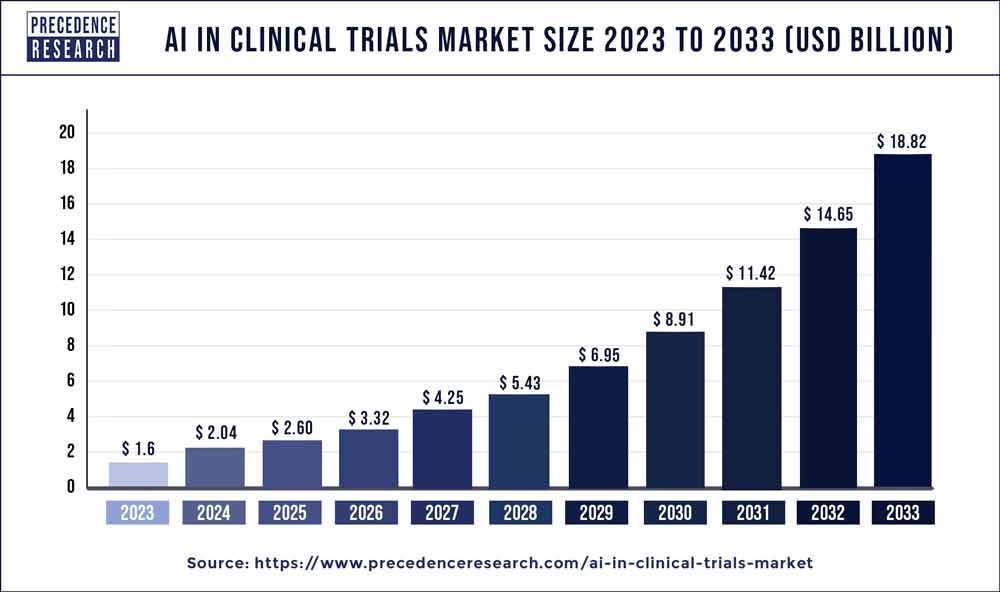
The global AI in clinical trials market size is projected to hit around USD 18.82 billion by 2033, poised to grow at a CAGR of 28% from 2024 to 2033.
Key Points
- North America held the dominating share of the AI in clinical trials market in 2023.
- By offering, the services segment held the largest market share in 2023.
- By technology, the deep learning segment held the dominating market share in 2023.
- By application, the infectious disease segment dominated the market in 2023.
- By end-user, the pharmaceutical segment held the largest share of the market in 2023; the segment is observed to sustain dominance throughout the forecast period.
Introduction:
The integration of Artificial Intelligence (AI) in clinical trials has marked a significant paradigm shift in the pharmaceutical and healthcare industries. AI technologies, including machine learning, natural language processing, and predictive analytics, are revolutionizing the way clinical trials are conducted, monitored, and analyzed. This transformation offers unprecedented opportunities for enhancing the efficiency, accuracy, and cost-effectiveness of clinical research processes, ultimately leading to accelerated drug development and improved patient outcomes.
Growth Factors:
Several key factors are driving the growth of the AI in clinical trials market. Firstly, the increasing volume and complexity of clinical trial data necessitate advanced analytical tools to extract meaningful insights efficiently. AI algorithms excel in handling vast datasets, identifying patterns, and predicting outcomes, thereby streamlining data analysis and decision-making processes. Moreover, the rising demand for personalized medicine and targeted therapies requires tailored approaches to clinical trial design and patient stratification, where AI-driven precision medicine solutions play a pivotal role.
Additionally, the growing emphasis on real-world evidence and decentralized clinical trials (DCTs) in response to the COVID-19 pandemic has fueled the adoption of AI technologies. These innovations enable remote data collection, virtual monitoring, and patient engagement, enhancing trial accessibility, inclusivity, and flexibility while mitigating operational challenges and geographical barriers. Furthermore, collaborations between pharmaceutical companies, technology firms, and research institutions are fostering the development of AI-powered platforms and solutions tailored to specific therapeutic areas, accelerating innovation and market expansion.
AI in Clinical Trials Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 28% |
| Global Market Size in 2023 | USD 1.6 Billion |
| Global Market Size by 2033 | USD 18.82 Billion |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Offering, By Technology, By Application, and By End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
AI in Clinical Trials Market Dynamics
Driver:
One of the primary drivers propelling the uptake of AI in clinical trials is its potential to enhance operational efficiency and reduce time-to-market for new therapies. Traditional clinical trial processes are often time-consuming, resource-intensive, and prone to inefficiencies, leading to significant delays and escalating costs. By automating repetitive tasks, optimizing protocol designs, and facilitating predictive modeling, AI systems enable faster patient recruitment, more accurate endpoint assessments, and proactive risk management, thereby expediting trial timelines and minimizing development costs.
Moreover, AI-driven predictive analytics and biomarker discovery facilitate more precise patient stratification and treatment selection, enhancing trial outcomes and increasing the likelihood of successful drug development. This predictive power enables researchers to identify relevant patient populations, predict treatment responses, and anticipate adverse events, enabling proactive intervention and personalized care delivery. Additionally, AI-powered data analytics enhance the quality and integrity of clinical trial data, ensuring compliance with regulatory standards and facilitating seamless collaboration across stakeholders.
Restraint:
Despite the promising opportunities offered by AI in clinical trials, several challenges and limitations must be addressed to realize its full potential. One of the primary concerns is the lack of standardization and regulatory guidance surrounding AI-based algorithms and predictive models in the context of clinical research. The variability in data sources, model architectures, and validation methods can lead to inconsistencies, biases, and uncertainties in AI-driven analyses, raising questions regarding reliability, reproducibility, and regulatory compliance.
Furthermore, the integration of AI technologies into existing clinical trial workflows requires substantial investments in infrastructure, resources, and training. Many organizations face challenges in implementing and scaling AI solutions due to limited expertise, data interoperability issues, and cultural barriers. Additionally, concerns regarding data privacy, security, and ethical considerations pose significant hurdles to the widespread adoption of AI in clinical trials, particularly concerning patient consent, data ownership, and algorithmic transparency.
Opportunity:
Despite these challenges, the growing momentum towards digital transformation and innovation in healthcare presents significant opportunities for the expansion and evolution of the AI in clinical trials market. Regulatory agencies, including the FDA and EMA, are increasingly embracing AI technologies and promoting regulatory frameworks to facilitate their integration into clinical research practices. Collaborative initiatives such as the FDA’s Digital Health Innovation Action Plan and the European Commission’s AI in Health Strategy aim to accelerate the development and adoption of AI-driven solutions while ensuring patient safety, data integrity, and regulatory compliance.
Moreover, advancements in AI algorithms, computational technologies, and data analytics are driving the development of more sophisticated and reliable AI-driven tools for clinical trial optimization, patient monitoring, and predictive modeling. Innovations such as federated learning, explainable AI, and blockchain-enabled data sharing hold promise for addressing key challenges related to data privacy, model interpretability, and decentralized collaboration in clinical research. Additionally, the emergence of AI-powered virtual assistants, chatbots, and telemedicine platforms is transforming patient engagement, recruitment, and retention strategies, enhancing the overall efficiency and patient-centricity of clinical trials.
Read Also: Network Engineering Services Market Size to Attain $97 Bn by 2033
Recent Developments
- In September 2023, a global pioneer in providing individuals and institutions with reliable intelligence to change the world, Clarivate Plc announced the creation of an Academia & Government Innovation Incubator. This will quicken its approach to fostering creativity, using AI, and launching cutting-edge products for its academic clients and users.
- In July 2023, by advancing the first medication identified and created by generative AI into Phase II clinical trials involving humans, Insilico Medicine has set a new standard in artificial intelligence drug research. The primary program, INS018_055, is a pan-fibrotic inhibitor that may be the first of its kind. Insilico’s moonshot medication unequivocally proves the viability of the company’s end-to-end AI drug development platform, Pharma. AI.
AI in Clinical Trials Market Companies
- AiCure
- Antidote Technologies
- Deep 6 AI
- Mendel.ai
- Phesi
- Saama Technologies
- Signant Health
- Trials.ai
- Innoplexus
- IQVIA
- Median Technologies
- Medidata
Segments Covered in the Report
By Offering
- Software
- Services
By Technology
- Machine learning
- Deep learning
- Supervised
By Application
- Cardiovascular
- Metabolic
- Oncology
- Infectious diseases
By End-user
- Pharma
- Biotech
- CROs
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/